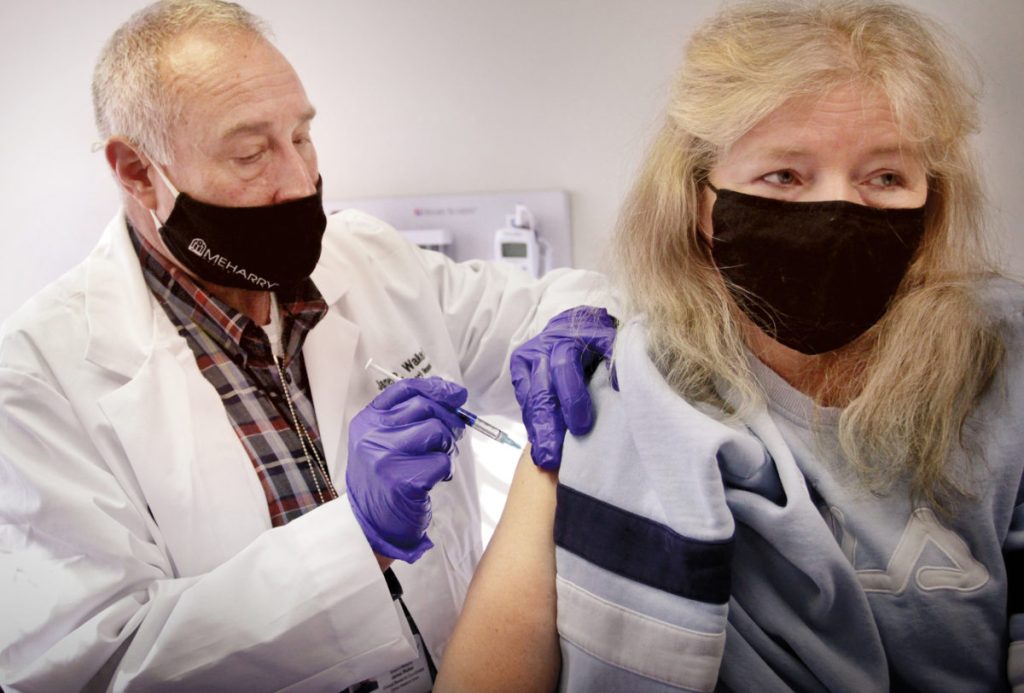
NASHVILLE, TN — Meharry Medical College is kicking off the new year with the launch of its Novavax COVID-19 vaccine clinical trial. Enrollment for the trial began on Monday, January 4 as 12 individuals received the first injection of the trial.
Participants in the Novavax trial at Meharry will receive the first injection upon enrollment, and can expect to receive the second injection 21 days later. All participants will be monitored over a 26-month period. Meharry hopes to enroll at least 250 participants in the trial, and recruitment is ongoing. Priority enrollment will be given to minority participants with comorbid conditions.
“We are thrilled to begin enrollment for the Novavax COVID-19 vaccine trial at Meharry,” said Dr. Rajbir Singh, co-principal Investigator of the clinical trial and interim director of the Clinical and Translational Research Center at Meharry. “For more than a century, minority groups have been left out of healthcare, leading to years of misdiagnosis and mistreatment. This clinical trial puts minority individuals front and center to ensure the vaccine is efficacious for communities who have been historically underserved. Black and brown people must be represented rather than overlooked, which is why Meharry is honored to oversee this research and establish the best possible outcome for minority populations.”
This trial is an important step toward protecting the community against COVID-19, a virus that has disproportionately impacted minority communities since the first cases were detected. Though multiple COVID-19 vaccines are expected to be available by the middle of this year, Dr. Singh is encouraging all community members to remember to stay vigilant.
“Even as vaccines are available and trials continue, we must not let our guard down, “said Dr. Singh. “We must continue to practice safety measures that we know will keep ourselves and our family members safe including wearing masks, washing our hands and social distancing.”
The launch of the Novavax trial is just one piece of Meharry’s comprehensive fight against COVID-19. To date, the College’s ongoing COVID efforts include managing Metro Nashville’s three drive-through testing sites, working with community partners to provide weekend testing at area churches, educating the Nashville community on how to best protect themselves and more.
Just last month, Meharry President and CEO Dr. James Hildreth served as a temporary voting member of the U.S. Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review both the Pfizer and Moderna vaccine candidates. Both candidates were subsequently recommended by the Committee for emergency use authorization.
For more information on this trial, how to register or Meharry’s ongoing efforts to fight COVID-19, visit https://home.mmc.edu/covid-19trial/.